
If FDA declines to approve pimavanserin for this indication, there is the option for a subsequent confirmatory study to better understand specific benefits of this drug, which is already approved to treat psychosis in Parkinson’s disease. Medications such as pimavanserin affect brain receptors differently from historical treatments such as antipsychotic drugs and may provide greater benefit without the challenging side effects of antipsychotics. However, in a broader context there are some positive things happening. So, what happens now? FDA will further review the evidence, with non-binding consideration of the Advisory Committee’s vote and comments and will come to a decision over the next couple months. Thus, there is some uncertainty and even the Advisory Committee experts did not reach consensus on the question, “Is the drug effective for psychosis in Alzheimer’s disease.” However, the impact in the Alzheimer’s group specifically was less robust and not statistically significant, although the study was not designed to assess the Alzheimer’s group independently. The results showed that pimavanserin treatment substantially reduced psychosis relapse over a six-month period in the overall group with dementia. A more recent study evaluated whether pimavanserin could help prevent recurrence of psychosis among those with dementia who had successfully responded to initial treatment with this drug. The evidence that was presented showed that six weeks treatment with pimavanserin was statistically better than placebo treatment among nursing home residents with Alzheimer’s disease and delusions or hallucinations, although the magnitude of benefit was small and there were some concerns regarding the design and conduct of the study. The pharmaceutical company developing pimavanserin, Acadia, subsequently submitted a revised request for approval for psychosis in Alzheimer’s disease, specifically, which was the subject of last week’s Advisory Committee review. At that time, the Agency felt that the response to treatment differed across the dementia subgroups and thus did not support the broad indication for “psychosis in dementia”. This input comes on the heels of an FDA review last year that declined to approve pimavanserin for psychosis in a broad group of dementia syndromes, including Alzheimer’s disease, vascular dementia, Lewy body and Parkinson’s disease dementia, and frontotemporal dementias. The Committee voted 9 to 3, with the majority finding insufficient evidence of effectiveness in this population. Assessment of Cognitive Complaints Pocket GuideĬontributed by David Sultzer, MD and Joshua Grill, PhDĪn FDA Advisory Committee met on June 17 to provide input to the Agency regarding the effectiveness of pimavanserin for the treatment of psychosis in Alzheimer’s disease.Assessment of Cognitive Complaints Toolkit.SoCal Alzheimer’s Disease Research Conference.Outreach, Recruitment, and Engagement Core.Hallucinations Lewy body disease Parkinson’s disease psychosis treatment. Unfortunately, current therapies for hallucinations offer only limited benefits and future research efforts are desperately required to improve the management of these challenging symptoms. Antidepressants may be beneficial in the appropriate setting. Refractory or severe symptoms may require the cautious use of atypical antipsychotics.

For well-formed visual hallucinations, acetylcholinesterase inhibitors are recommended first-line. Screening, education, medication review and the avoidance of common triggers are important.
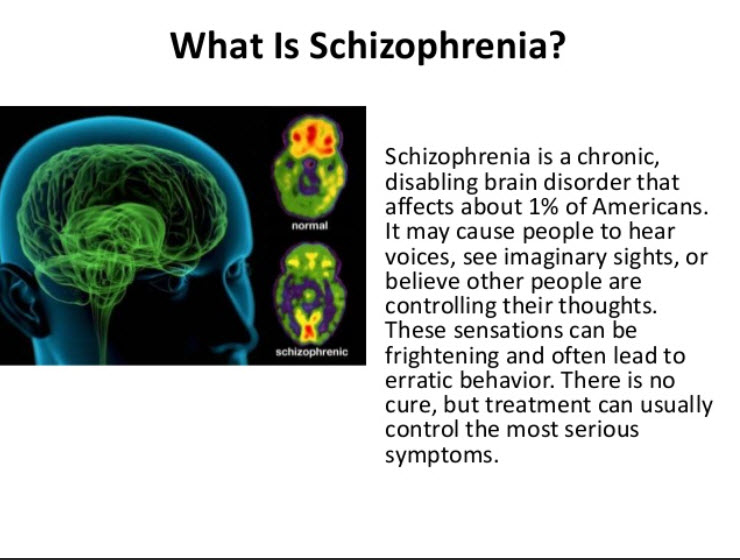

Treatment of hallucinations in Parkinson's disease should be both individualized and multifaceted. This review aims to examine current evidence for the management of hallucinations in Parkinson's disease. Hallucinations in Parkinson's disease are common, can complicate medication management and significantly impact upon the quality of life of patients and their carers.


 0 kommentar(er)
0 kommentar(er)
